-
题目: 未知类型
在25℃、101 kPa下,1 g甲醇( CH3OH)燃烧生成CO2和液态水时放热22.68 kJ,下列热化学方程式正确的是( ) A.CH3OH(l)+ 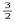 O2(g)===CO2(g)+2H2O(l);ΔH="+725.8" kJ/mol
O2(g)===CO2(g)+2H2O(l);ΔH="+725.8" kJ/molB.2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-1452 kJ/mol C.2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-725.8 kJ/mol D.2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH="+1452" kJ/mol 答案不对?请尝试站内搜索